The Inflamed Brain: How inflammation can affect mood and behaviour
- Etta Nettis
- Oct 7, 2020
- 6 min read
Updated: Oct 4, 2023
Think of the last time you had a cold sore, or the flu, or a bowel infection…I am sure you don’t have the fondest memories of that experience, as your mood must have been quite bad.
We are inclined to think that having a low or irritable mood during sickness, as well as experiencing fatigue, disrupted sleep or decreased appetite, is a natural reaction to physical distress. In other words, it is obvious to feel low when your body is in pain.
However, science has started to show that there is a precise biological mechanism explaining the connection between body and mind, physical illness and depressed-like behaviour. I am psychiatrist and a PhD student, and my research aims to study this connection.
Indeed, sickness behaviour, which is the behavioural response to physical illness, is a complex reaction involving the immune system and its effects on the brain.
The role of the immune system in mood regulation has been the focus of several blogs here on InSPIre the mind, but it can be studied in so many different ways, and some of you might not be our regular readers. So, I will summarise below what the immune system does, how it can affect the brain and why this is important for research in psychiatry.
What is the role of the immune system?
The immune system is a complex network of organs, tissues, cells (mainly the immune cells) and the substances that they produce. Its main role is to defend our body from infections, like an army of soldiers, with an overall defensive strategy called inflammation.
How does the immune system affect the brain?
When inflammation is triggered, a series of proteins — the inflammatory cytokines — are released in the blood stream and in the tissues, acting like messengers between different organs, with the aim of alerting the body to fight the infection.
What has become clear in recent years, is that inflammatory cytokines can also reach the brain and affect some crucial cellular processes involved in mood and behaviour regulation.
This has been suggested by animal studies, showing that cytokines can reach the brain via the blood-brain barrier (BBB), a border of cells protecting the brain against circulating toxins (like those in the picture below) and, at the same time, allowing vital nutrients and other substances to pass. These animal studies indicated that cytokines are among those substances that can go through!
Once in the brain, inflammatory cytokines can alert the brain cells belonging to the immune system, like microglia and astrocytes. Once alerted, these cells go through changes in shape and dimension, in order to reach their activated state and produce more inflammatory mediators, leading to a general condition of brain inflammation.
This, in turn, can have disruptive consequences on neuron generation and functioning in different brain areas, such as the hippocampus, involved in the modulation of emotions, motivation, learning and memory. Finally, this is followed by the development of depressive-like behaviour.
The whole journey of inflammatory cytokines (like Interleukin-6 –[IL-6] and Tumor Necrosis Factor-alpha [TNF-a]) is illustrated in the picture below and is described more in detail in a recent scientific chapter that I have written.
The good news is that today it is possible to study changes in the structure and functioning of brain cells of living humans (something we call ‘in vivo’), thanks to a technique called Positron Emission Tomography (PET). This is an imaging technique, involving a small dose of radiation, that can provide us with pictures of the human brain and information about changes in cells activity, including cells like microglia that are involved in brain inflammation. So, we can now explore whether humans have inflammation in their brain by using PET.
Why is this important?
If inflammation can go from the body to the brain and lead to the development of depressive symptoms, this could explain why, when we have an infection, we also feel so low!
This would also explain why a high proportion of people with chronic inflammatory conditions (for example, Rheumatoid Arthritis) tend to suffer from comorbid depression.
Therefore, in those circumstances, we could treat depressive symptoms with alternative strategies, like anti-inflammatories.
Now, in order to support this hypothesis, imagine if it was possible to track the theory of sickness behaviour step by step, following inflammation in its journey from beginning to end, from the body to the brain, by recreating conditions “artificially”?
Well, that’s were my research and my PhD come in. With the help of some healthy volunteers and of PET imaging, I had the opportunity to follow the journey of inflammation and discover what it really does to the brain.
The FLAME project and the journey from the body to the brain
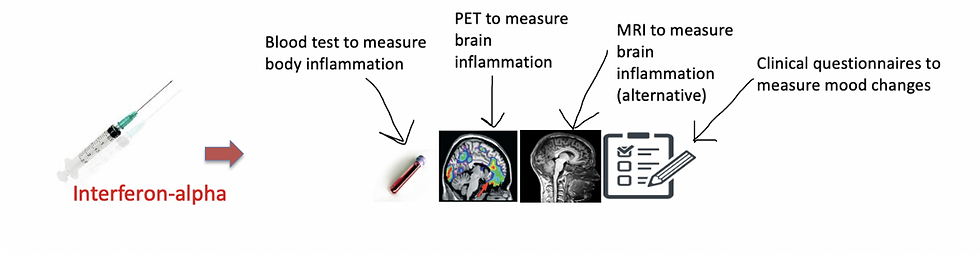
In my study, called FLAME (because it involves inflammation), I recreated the conditions of inflammation in the bodies of 7 healthy male volunteers, by administering a drug called Interferon-alpha. This drug can challenge the immune system to produce a temporary state of inflammation, which lasts up to 72 hours. Don’t worry, Interferon-alpha is quite safe, and one injection can only cause some flu-like symptoms which can be easily treated with paracetamol (the study was approved by an ethical committee and all participants gave their consent to take part before starting. The study did not compromise their health in any possible way).
After the injection of interferon-alpha, I measured the presence of body inflammation with blood tests. I also measured the development of brain inflammation with PET imaging and with an alternative technique called Magnetic Resonance Imaging (MRI). This is considered to be less specific than PET in detecting brain inflammation, but it is much cheaper and less invasive for participants (since there is no radiation involved), so I wanted to explore its potential too. Finally, I measured temporary mood changes with some clinical questionnaires.
Overall, I wanted to test whether the temporary inflammation caused in the body by Interferon-alpha was associated with inflammation in the brain and with some temporary mood changes. A more detailed illustration of the different measurements is shown below.
Just as in my favourite movies, research can have twists in the plot, like events or conclusions that I did not expect at the start.
This was the case also for the FLAME study. I found that interferon-alpha was causing increased inflammation in the body of participants, as well as creating some temporary mood changes, like increased fatigue and low mood, from 4 to 24 hours after the injection. Overall, this reflected my initial expectations.
However, PET imaging gave different results from what I anticipated. It looked like there was no inflammation in the brain as brain cells involved in inflammation did not show particular activity. I later realized, that there was a methodological limitation in the PET technique that was used. Indeed, PET was not sensitive enough to measure changes in the structure and functions of microglia, the most important brain immune cells, under these specific experimental conditions. So, I could not really tell whether the inflammation caused by Interferon-alpha was able to go from the body to the brain.
This indicated that future studies using PET imaging should be using some precautions when interpreting their results. My data was published in a scientific journal that considered them useful for future research and you can read the full article here.
Just as I was ready to think that perhaps inflammation did not reach the brain, I thought that perhaps the problem was the imaging approach. You know when you go to the theatre and, for some reason, the leading actor cannot perform that day? They send the understudy on stage. Well, sometimes the understudy performs even better than the leading actor, and you leave the theatre surprised and happy to have discovered a new talent. A similar thing might have happened to me in this experiment.
The MRI technique, which I employed as the neuroimaging understudy to PET, might be able to show brain structure after Interferon-alpha, which could indicate the presence of brain inflammation. As soon as I have more data on this, I will write another blog!
My PhD research showed that even if results are not exactly as expected, they can still provide new perspectives of study and make way for future work on the same topic, in this case involving either more precise PET techniques or also MRI, which is cheaper and safer, to study inflammation in the brain.
You may wonder: how is this research useful for people? Well, this is the focus of another project that I conducted, regarding the possibility to treat depressive symptoms with anti-inflammatory drugs, which can have an effect on brain inflammation.
But this is a story that I will tell you next time, and of course, there will be a twist!












